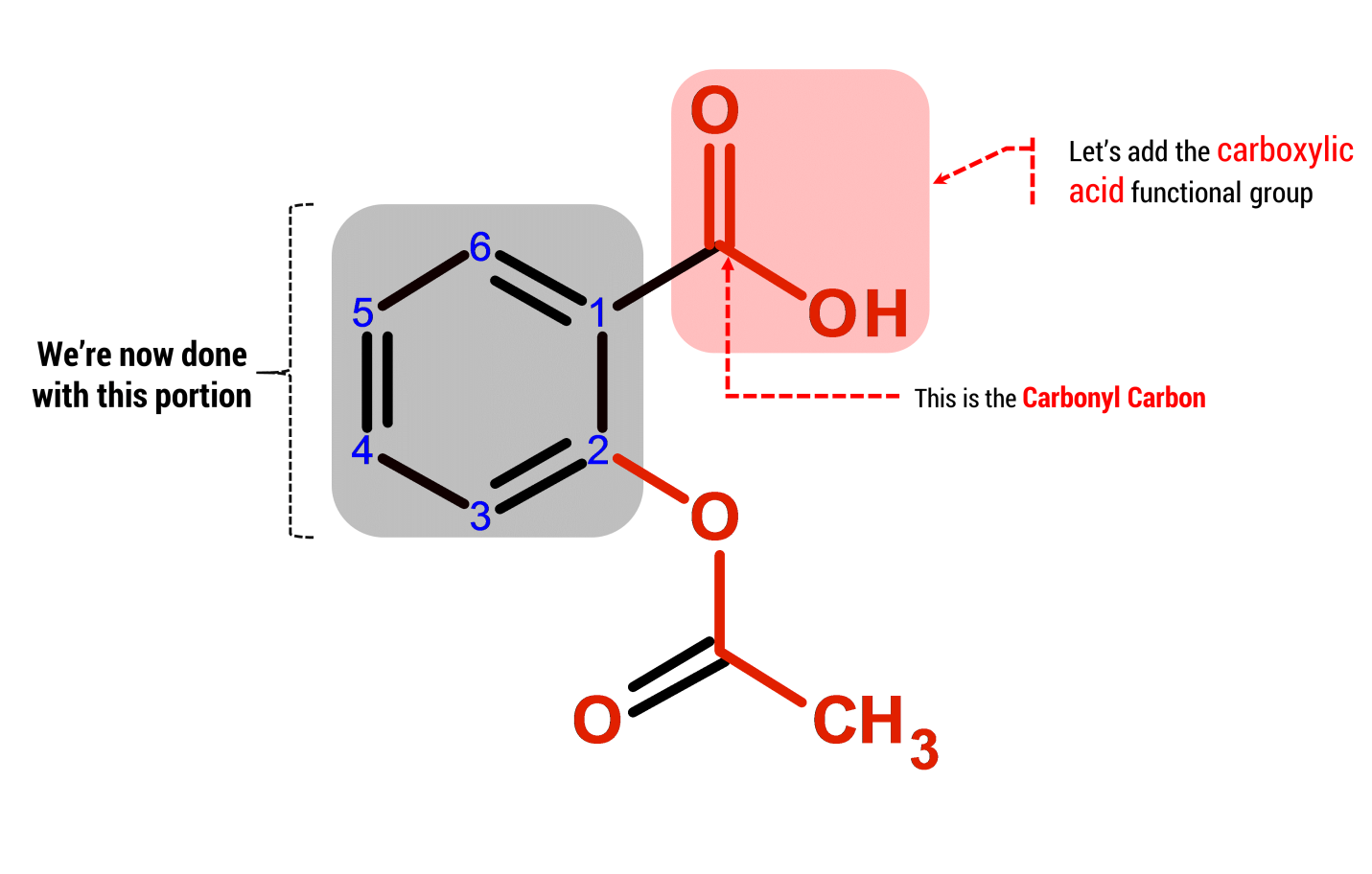
Focus on the carboxyl (-COOH) and ester (-COO-) groups: these are the key players determining aspirin’s properties. The carboxyl group contributes to aspirin’s acidity, influencing its solubility and how it interacts within the body. Understanding its behavior is critical for comprehending aspirin’s pharmacological action.
The ester group, formed during aspirin synthesis from salicylic acid and acetic anhydride, is equally important. This specific functional group is responsible for aspirin’s relatively rapid hydrolysis in the body, converting it back into salicylic acid, the active pain-relieving component. This rapid conversion impacts its duration of action and overall efficacy.
Careful consideration of both functional groups reveals the intricate structure-activity relationship of aspirin. The presence and interaction of the carboxyl and ester groups dictate how aspirin is absorbed, metabolized, and ultimately exerts its therapeutic effects. A clear understanding of these functional groups provides a solid foundation for deeper investigation into aspirin’s chemistry and pharmacology.
- Functional Groups in Aspirin
- Carboxylic Acid Group (-COOH)
- Ester Group (-COO-)
- Impact of Functional Groups on Aspirin’s Properties
- Synthesis Considerations
- Identifying the Carboxylic Acid Group in Aspirin
- Understanding the Ester Functional Group in Aspirin
- The Role of Functional Groups in Aspirin’s Pharmaceutical Activity
- Comparing Aspirin’s Functional Groups to Related Compounds
Functional Groups in Aspirin
Aspirin contains two key functional groups: a carboxylic acid and an ester.
Carboxylic Acid Group (-COOH)
The carboxylic acid group is responsible for aspirin’s acidic properties. This group readily donates a proton (H+), making aspirin a weak acid. This acidity influences its solubility and how it interacts with the body.
Ester Group (-COO-)
The ester group is formed during aspirin synthesis. It’s the product of an esterification reaction between salicylic acid and acetic anhydride. This specific ester linkage is crucial for aspirin’s pharmacological activity.
Impact of Functional Groups on Aspirin’s Properties
The presence of both the carboxylic acid and ester significantly affects aspirin’s behavior. The carboxylic acid contributes to its solubility in slightly basic environments, such as the intestines, improving absorption. The ester group modifies the properties of salicylic acid, making aspirin less irritating to the stomach than salicylic acid itself.
| Functional Group | Chemical Formula | Impact on Aspirin |
|---|---|---|
| Carboxylic Acid | -COOH | Acidity, solubility, interaction with the body |
| Ester | -COO- | Pharmacological activity, reduced stomach irritation |
Synthesis Considerations
Understanding these functional groups is fundamental to aspirin synthesis. The esterification reaction specifically targets the hydroxyl group (-OH) of salicylic acid, replacing it with the acetate group (-COCH3) to form the ester. This transformation significantly alters the drug’s properties.
Identifying the Carboxylic Acid Group in Aspirin
Look for the -COOH functional group. This carboxyl group is the key identifier of a carboxylic acid.
Aspirin’s chemical structure clearly shows this group attached to a benzene ring. The carbon atom is double-bonded to one oxygen and single-bonded to a hydroxyl group (-OH).
Infrared (IR) spectroscopy readily detects the C=O stretch (around 1700 cm⁻¹) characteristic of the carbonyl group within the carboxyl group. You’ll also see a broad O-H stretch (around 3000 cm⁻¹) due to the hydroxyl group, further confirming the presence of the carboxylic acid.
NMR spectroscopy offers additional evidence. The carboxylic acid proton (-COOH) typically appears downfield (around 10-13 ppm) in the ¹H NMR spectrum, distinct from other protons in the molecule. The carbon of the carbonyl group (C=O) shows up at a characteristic chemical shift in the ¹³C NMR spectrum, usually around 165-185 ppm.
Titration with a strong base, like sodium hydroxide, will neutralize the carboxylic acid group, creating a salt and allowing you to determine the quantity of the acid present. This reaction supports the presence of the acidic carboxylic acid group.
Understanding the Ester Functional Group in Aspirin
Aspirin’s activity hinges on its ester functional group. This group, specifically an acetyl group (-COCH3) bonded to a salicylic acid molecule, is responsible for its pain-relieving and anti-inflammatory properties.
The ester linkage is formed through a reaction between the carboxylic acid group of acetic acid and the hydroxyl group of salicylic acid. This reaction removes a water molecule, creating the characteristic ester bond (-COO-).
Hydrolysis, the reverse reaction, breaks this ester bond. This process is relevant because it describes how aspirin is metabolized in the body. Enzymes in the body break down the ester, producing salicylic acid, which is the active form of the drug responsible for its therapeutic effects.
The presence of the ester is crucial for the molecule’s pharmaceutical action. Without this specific functional group, aspirin would lose its effectiveness. The acetyl group modifies the properties of salicylic acid, making it less irritating to the stomach while retaining analgesic properties.
Analyzing the ester’s role helps us understand aspirin’s mechanism of action and the importance of its chemical structure in its therapeutic application. Knowing this helps to predict the drug’s behavior and how it interacts with the human body. The ester group’s structure directly influences how effectively the drug interacts with its target sites.
The Role of Functional Groups in Aspirin’s Pharmaceutical Activity
Aspirin’s effectiveness stems directly from its specific functional groups. The carboxylic acid group (-COOH) contributes to its acidity, allowing it to readily dissolve in the stomach’s slightly acidic environment. This solubility is critical for absorption into the bloodstream.
The ester group (-COO-) links the acetyl group to the salicylic acid moiety. This ester linkage is crucial; its hydrolysis by enzymes in the body releases salicylic acid, the active pain-relieving and anti-inflammatory component. The rate of hydrolysis influences the duration of aspirin’s action.
The phenolic hydroxyl group (-OH) on the salicylic acid portion is responsible for aspirin’s primary pharmacological effects. This group interacts with cyclooxygenase (COX) enzymes, inhibiting prostaglandin synthesis. This inhibition reduces inflammation, pain, and fever.
The benzene ring provides structural rigidity and influences the interactions of aspirin with its target enzymes. Its hydrophobic nature contributes to aspirin’s ability to cross cell membranes.
In short, the interplay of the carboxylic acid, ester, phenolic hydroxyl, and benzene ring dictates aspirin’s solubility, metabolism, and interaction with COX enzymes, resulting in its analgesic, antipyretic, and anti-inflammatory properties.
Comparing Aspirin’s Functional Groups to Related Compounds
Aspirin (acetylsalicylic acid) possesses two key functional groups: a carboxylic acid (-COOH) and an ester (-COO-). Understanding how these groups compare to similar groups in related compounds reveals much about aspirin’s properties and mechanism of action.
Let’s examine similar compounds:
- Salicylic acid: This differs from aspirin only by lacking the acetyl group. The absence of the acetyl group significantly increases salicylic acid’s acidity and irritating properties. This difference highlights the ester group’s role in modifying the carboxylic acid’s behavior, making aspirin less harsh on the stomach.
- Acetic acid (vinegar): This possesses only the carboxylic acid group. Comparing its properties (e.g., acidity, odor) to aspirin’s illustrates the influence of the ester and benzene ring on the overall compound properties.
- Methyl salicylate (oil of wintergreen): This compound features a methyl ester instead of the acetyl ester found in aspirin. This change alters its pharmacological activity and smell, demonstrating the impact of even subtle structural variations on functional properties.
A tabular comparison helps summarize these differences:
| Compound | Carboxylic Acid | Ester | Other Relevant Groups | Key Properties |
|---|---|---|---|---|
| Aspirin | Present | Acetyl ester | Benzene ring | Analgesic, anti-inflammatory, antipyretic |
| Salicylic acid | Present | Absent | Benzene ring | More acidic, irritating |
| Acetic acid | Present | Absent | None | Highly acidic, pungent odor |
| Methyl salicylate | Present | Methyl ester | Benzene ring | Distinct odor, topical analgesic |
By analyzing these comparisons, we gain a clearer understanding of how specific functional groups contribute to the unique pharmacological profile of aspirin.