Begin by carefully reviewing your patient’s medical history, focusing on any known allergies or previous adverse reactions to phenytoin. A complete blood count (CBC) and liver function tests (LFTs) should be obtained prior to initiating treatment.
The recommended initial IV Dilantin loading dose is 15-20 mg/kg, administered slowly at a rate not exceeding 50 mg/minute. Closely monitor the patient for signs of hypotension or cardiac arrhythmias during infusion. Continuous ECG monitoring is crucial during the loading phase.
Once the loading dose is complete, transition to a maintenance infusion. Dosage adjustments are necessary based on serum phenytoin levels and clinical response; target therapeutic range is typically 10-20 mcg/mL. Regular serum phenytoin level monitoring is paramount to ensure efficacy and avoid toxicity.
Remember: Dilantin’s IV formulation is formulated in propylene glycol, so rapid infusion carries risks. Use a large-gauge IV catheter and monitor the infusion site for complications like phlebitis. Patients exhibiting signs of toxicity – including nystagmus, ataxia, or confusion – require immediate attention and possible dosage reduction.
Always consult current clinical guidelines and your institution’s specific protocols before administering IV Dilantin. This information serves as a starting point, not a comprehensive treatment plan.
- Iv Dilantin Protocol: A Detailed Guide
- Understanding the Indications for IV Dilantin Administration
- Preparing and Administering IV Dilantin: A Step-by-Step Guide
- Monitoring Patients Receiving IV Dilantin: Key Parameters and Precautions
- Managing Potential Adverse Effects of IV Dilantin Therapy
- Discontinuing IV Dilantin: A Safe and Effective Approach
- Monitoring Serum Levels
- Transition to Oral Medication
- Recognizing Withdrawal Symptoms
- Patient Education and Support
- Documentation
Iv Dilantin Protocol: A Detailed Guide
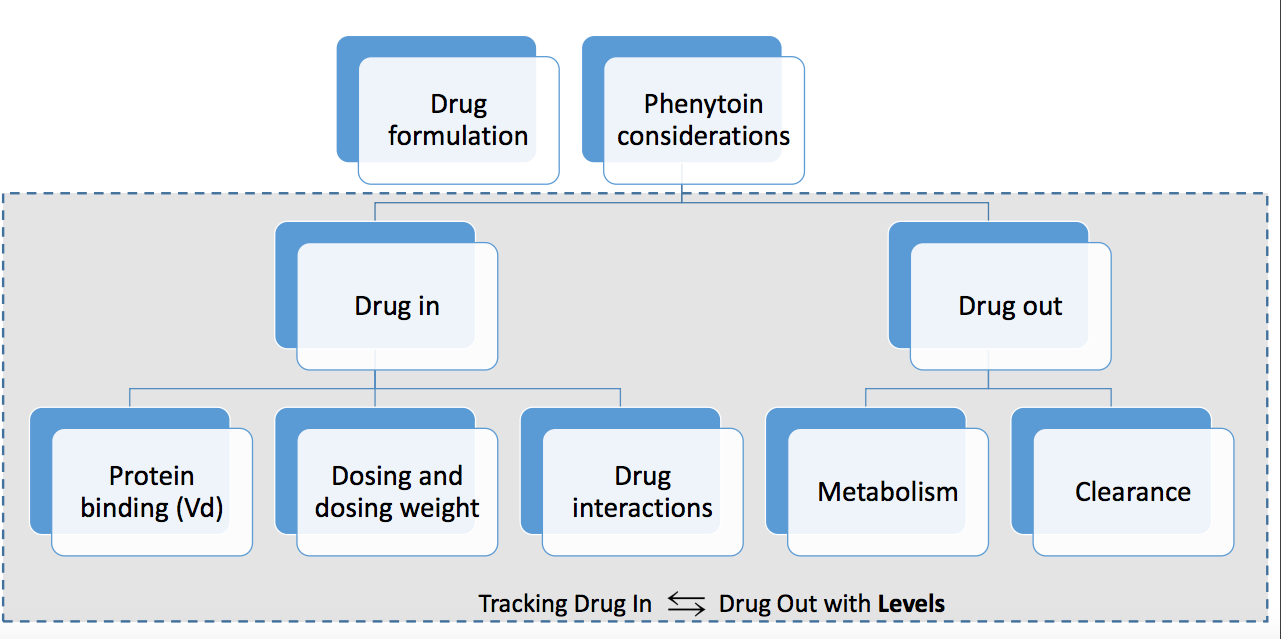
Always verify the patient’s weight accurately before initiating the infusion. This ensures precise dosage calculation. Dilantin (phenytoin) is highly protein-bound; consider this factor in patients with hypoalbuminemia, requiring potential dosage adjustments.
Administer Dilantin intravenously slowly, at a rate not exceeding 50 mg/minute. Rapid infusion can cause hypotension and cardiac arrhythmias. Use a filtered intravenous line to prevent particle infusion.
Monitor the patient’s blood pressure and ECG continuously during and for at least 30 minutes after the infusion. Watch for signs of hypotension or arrhythmias. These are common adverse effects requiring prompt intervention.
Dilantin is incompatible with many intravenous solutions. Use only compatible solutions, such as normal saline, per your hospital’s formulary. Improper mixing can precipitate drug crystallization.
Observe the patient closely for signs of toxicity such as nystagmus, ataxia, and slurred speech. These symptoms necessitate immediate intervention and may require dosage reduction.
Obtain serum phenytoin levels regularly to ensure therapeutic drug levels are maintained and avoid toxicity. Dosage adjustments are often based on these levels.
Maintain adequate hydration. This helps prevent the adverse effects associated with Dilantin administration and improves drug clearance. Monitor urine output.
Document all aspects of Dilantin administration carefully, including dose, infusion rate, time, any adverse effects observed, and laboratory results. Accurate record-keeping is vital for patient safety.
Remember: This guide provides general information. Always consult your institution’s specific protocol and follow your physician’s orders. Strict adherence to the established guidelines is paramount for patient safety.
Understanding the Indications for IV Dilantin Administration
IV Dilantin (phenytoin) is primarily used for managing acute seizures, specifically status epilepticus. This life-threatening condition requires immediate intervention.
Beyond status epilepticus, IV Dilantin finds application in preventing seizures following certain neurological injuries, such as traumatic brain injury. Its use here aims to reduce the risk of post-traumatic seizures.
Certain types of epilepsy may also warrant IV Dilantin administration, particularly when oral medication is ineffective or impractical. This includes cases where rapid seizure control is needed.
However, remember IV Dilantin isn’t a first-line treatment for all seizure types. The physician will carefully assess the patient’s condition and seizure history before choosing the most appropriate treatment.
| Indication | Description |
|---|---|
| Status Epilepticus | Life-threatening seizure lasting longer than 5 minutes or recurring seizures without recovery of consciousness. |
| Post-Traumatic Seizures | Preventing seizures after traumatic brain injury. |
| Refractory Epilepsy | Epilepsy unresponsive to other treatments. |
Always consult prescribing information and follow the physician’s orders precisely. Individual patient factors influence the appropriateness of IV Dilantin.
Preparing and Administering IV Dilantin: A Step-by-Step Guide
Always verify the physician’s order and patient allergies before proceeding. Ensure you have the correct Dilantin (phenytoin) concentration and dosage.
- Prepare the IV solution: Dilute the phenytoin in accordance with the manufacturer’s instructions. Typically, this involves adding the Dilantin vial to a compatible intravenous solution like normal saline (0.9% NaCl) or dextrose 5% in water (D5W). Never use D5W with phenytoin sodium.
- Inspect the solution: Carefully examine the solution for any particulate matter or discoloration before administration. Discard if any abnormalities are present.
- Choose the appropriate IV tubing: Use an in-line filter to prevent any microparticles from entering the patient’s bloodstream. Use a suitable IV line and catheter. A larger gauge is preferable for faster infusion.
- Administer slowly: The maximum rate of infusion should not exceed 50 mg/minute. Faster rates increase the risk of hypotension and cardiovascular complications. Use an infusion pump for precise control and accurate infusion rate monitoring. Carefully monitor the patient for signs of adverse reactions.
- Monitor vital signs: Continuously observe the patient’s blood pressure, heart rate, and rhythm throughout the infusion. Report any significant changes to the physician immediately.
- Observe for side effects: Watch for signs of hypotension, arrhythmias, or other adverse reactions. These can include dizziness, nausea, or rash.
- Document carefully: Meticulously record the time of administration, dosage, and any observed side effects in the patient’s chart.
Important Considerations:
- Dilantin is incompatible with many intravenous solutions. Consult a drug compatibility chart for detailed information.
- Extravasation can cause significant tissue damage. Immediate discontinuation and appropriate management are necessary if extravasation occurs.
- Cardiac monitoring is often recommended, particularly in patients with pre-existing heart conditions.
This guide offers a general framework; always adhere to your institution’s policies and procedures. Consult your organization’s drug formulary and medication administration guidelines for complete and up-to-date information.
Monitoring Patients Receiving IV Dilantin: Key Parameters and Precautions
Closely monitor serum phenytoin levels. Target therapeutic range is generally 10-20 mcg/mL, but this can vary depending on the patient’s condition and seizure type. Frequent monitoring, particularly during the loading phase, is critical.
Regularly assess the patient’s neurological status. Note any changes in mental status, coordination, or motor function. These can be early indicators of toxicity or therapeutic failure.
- Observe for signs of nystagmus (rapid, involuntary eye movements).
- Monitor for ataxia (loss of coordination).
- Assess for slurred speech or cognitive impairment.
Carefully monitor cardiac rhythm. IV Dilantin can cause cardiac arrhythmias, including bradycardia. Continuous ECG monitoring during infusion is advisable, especially in patients with pre-existing cardiac conditions.
- Check blood pressure and heart rate frequently.
- Be prepared to manage potential arrhythmias.
Watch for hypotension. Dilantin can cause a drop in blood pressure, particularly during rapid infusion. Adjust the infusion rate accordingly and monitor blood pressure closely.
Administer IV Dilantin slowly to minimize the risk of adverse effects. Rapid infusion can increase the likelihood of hypotension, cardiac arrhythmias, and other complications. The recommended rate is generally no faster than 50 mg/minute.
Ensure proper hydration. Dilantin can exacerbate dehydration; maintain adequate intravenous fluids.
- Monitor urine output.
- Assess for signs of dehydration.
Regularly check complete blood counts (CBC) and liver function tests (LFTs). Dilantin can affect blood cell production and liver function, necessitating routine monitoring for potential complications.
Always be aware of potential drug interactions. Phenytoin interacts with numerous medications; review the patient’s medication list carefully. This includes interactions affecting metabolism and serum levels.
Managing Potential Adverse Effects of IV Dilantin Therapy
Closely monitor patients for hypotension, which can be managed with fluid resuscitation and potentially vasopressors if needed. Cardiac monitoring is recommended, especially during the initial infusion.
Observe for arrhythmias. Bradycardia or other rhythm disturbances require immediate attention and may necessitate adjustment of the infusion rate or the use of antiarrhythmic medications. Consult with a cardiologist as needed.
Nausea and vomiting are common. Antiemetics, such as ondansetron or promethazine, can effectively reduce these side effects. Consider administering antiemetics prophylactically, especially in patients with a history of nausea or vomiting.
Monitor for signs of gingival hyperplasia. Good oral hygiene, including regular brushing and flossing, can help minimize this risk. Regular dental check-ups are advised during long-term Dilantin therapy.
Watch for skin reactions, such as rash or Stevens-Johnson syndrome. Discontinue the infusion immediately if a serious rash develops and institute appropriate supportive care.
Serum Dilantin levels should be regularly monitored to ensure therapeutic levels are maintained while avoiding toxicity. Adjust the infusion rate based on these levels and the patient’s clinical response.
Always consult the prescribing information for complete details on potential adverse effects and management strategies.
Remember: Prompt recognition and management of adverse effects are key to patient safety and successful IV Dilantin therapy.
Discontinuing IV Dilantin: A Safe and Effective Approach
Gradually reduce the IV Dilantin infusion rate. A common approach involves decreasing the rate by 25-50 mg/minute every 12-24 hours, depending on the patient’s clinical response and seizure control. Closely monitor the patient’s serum Dilantin levels during this process.
Monitoring Serum Levels
Maintain therapeutic serum levels (typically 10-20 mcg/mL) throughout the tapering process. Frequent blood draws are necessary to ensure safe and controlled withdrawal. Adjust the reduction rate based on these levels. If levels fall below the therapeutic range, slow the tapering rate or temporarily maintain the current dosage.
Transition to Oral Medication
Once the IV infusion is discontinued, promptly transition the patient to oral Dilantin. The initial oral dose should closely match the final IV dose to maintain consistent drug levels. Continue to monitor serum levels and adjust the oral dose as needed.
Recognizing Withdrawal Symptoms
Seizures are a major concern during Dilantin withdrawal. Be prepared to manage potential breakthrough seizures with appropriate anticonvulsant medication. Observe for other potential symptoms, including nausea, vomiting, and dizziness. These symptoms may indicate a need to slow the withdrawal process.
Patient Education and Support
Provide the patient and their caregivers with thorough instructions regarding the medication regimen, potential side effects, and the importance of adherence to the prescribed schedule. Address any concerns and questions they may have. Offer ongoing support and monitoring.
Documentation
Meticulously document all aspects of the withdrawal process, including dosage adjustments, serum Dilantin levels, and any observed symptoms or adverse events. This record will be valuable for future reference and care.